Current Projects
Rigid electron rich PCP Pincer ligands for small molecule activation
Tridentate “pincer” ligands provide a versatile platform for tuning ligands to support transition metal-based catalysts. Using the Periodic Table as a tool box, the permutations of ligand arrays possible is limited only by the chemist’s imagination.
We have developed a family of electron-rich PCcarbeneP pincer ligands in which the donors are linked by rigid aromatic backbones. The carbenes are thus in that grey zone between Fischer and Schrock carbenes, and when coordinated to transition metals the carbene anchor of the pincer framework can become involved cooperatively in the reactivity of these compounds with small molecules.
We now have an extensive library of these ligands, evolved from the parent ligand in which the linker is a simple phenyl group, to ever more electron rich and rigid systems as shown in the graphic above.
The ligands can be attached to a variety of mid to late transition metals (Fe, Ru, Co, Rh, Ir, Ni, Pd, Pt) and current emphasis is on those of the first row transition series. Applications include activation and conversion of greenhouse gas molecules like N2O, CO2 and CH4; we are also interested in more conventional catalytic reactions using pincer metal compounds incorporating earth abundant metals.
Key papers:
LaPierre, E. A.; Piers, W. E.; Lin, J.-B.; Gendy, C. “Synthesis and structures of stable Pt(II) and Pt(IV) alkylidenes: evidence for π-bonding and relativistic stabilization” Chem. Eur. J. 2019, 25, 4305-4308.
LaPierre, E. A.; Piers, W. E.; Spasyuk, D. M.; Bi, W. “Activation of Si-H bonds across the nickel carbene bond in electron rich nickel PCcarbeneP pincer complexes.” Chem. Commun. 2016, 52, 1361-1364.
Doyle, L. E.; Piers, W. E.; Borau-Garcia, J.; Sgro, M. J.; Spasyuk, D. M. “Mechanistic Studies on the Addition of Hydrogen to Iridaepoxide Complexes with Subsequent Elimination of Water.” Chem. Sci. 2016, 7, 921-931.
Doyle, L. E.; Piers, W. E.; Borau-Garcia, J. “Ligand Cooperation in the Formal Hydrogenation of N2O Using a PCsp2P Iridium Pincer Complex.” J. Am. Chem. Soc. 2015, 137, 2187-2190.
Burford, R. J.; Piers, W. E.; Ess, D. H.; Parvez, M. “Reversible Interconversion Between a Monomeric Iridium Hydroxo and a Dinuclear Iridium m-Oxo Complex.” J. Am. Chem. Soc. 2014, 136, 3256-3263.
Gutsulyak, D. V.; Piers, W. E.; Parvez, M. Borau-Garcia, J. “Activation of Water, Ammonia and Other Small Molecules by PCcarbeneP Nickel Pincer Complexes.” J. Am. Chem. Soc. 2013, 135, 11776-11779.
Pentadentate ligand platforms for small molecule activation
Pentadentate tetrapodal ligands, like the neutral pentapyridyl framework (PY5) shown in the graphic above (introduced some time ago by Stack and Feringa), provide a solid platform for activation of small molecules by preventing complex decomposition through loss of the ligand trans to the reactive site. Complexes of M2+ ions of this ligand have recently been explored as proton reduction and water oxidation catalysts, but the neutral nature of the ligand renders all complexes of this ligand dicationic in nature.
We have incorporated borate functions into the chelate framework and incorporated pyrazolyl donors to generate the dianionic analog of PY5. Complexes from across the first row transition series can be made, including the early metals, and because ligand is dianionic, complexes with this ligand bear no charge. The ligand thus can be expected to stabilize high oxidation state compounds and study in non-polar media are possible.
We are exploring the coordination chemistry of this and related ligands to generate and study highly reactive metal oxo and imido compounds and develop catalysts for small molecule transformations. The toolbox of compounds we have made so far (M = Sc, V, Mn, Fe, Co, Ni) are also allowing us to explore well defined mixed-metal oxo compounds of general formula L5M-O-M’L5, where M ≠ M’.
Newer directions in this area include tetradentate ligand designs that incorporate the borate moiety and we envision active catalysts for N2O hydrogenation are plausible using these novel ligands.
Key papers:
Beh, D. W.; Piers, W. E.; Gelfand, B. S.; Lin, J.-B. “Tandem deoxygenative hydrosilation of carbon dioxide with a cationic scandium hydridoborate and B(C6F5)3” Dalton Trans. 2020, 49, 95-101.
Beh, D.; Piers, W. E.; del Rosal, I.; Maron, L., Gelfand, B. S.; Gendy, C.; Lin, J.-B. “Scandium alkyl and hydride complexes supported by a pentadentate diborate ligand: reactions with CO2 and N2O.” Dalton Trans. 2018, 47, 13680-13688.
Nurdin, L.; Spasyuk, D. M.; Fairburn, L.; Piers, W. E.; Maron, L. “Oxygen-oxygen bond cleavage and formation in Co(II) mediated O2 reduction processes via the potential intermediacy of a Co(IV) oxyl radical.” J. Am. Chem. Soc. 2018, 140, 16094-16105.
Nurdin, L.; Spasyuk, D. M.; Piers, W. E.; Maron, L.; Bi, D. W. “Reactions of Neutral Co(II) Complexes of a Dianionic Tetrapodal Pentadentate Ligand: Co(III) Amides from Imido Radicals.” Inorg. Chem. 2017, 56, 4157-4168.
Spasyuk, D. M.; Carpenter, S.; Kefalidis, C. E.; Piers, W. E.; Maron, L.; Neidig, M. L. “Facile Hydrogen Atom Transfer to Iron(III) Imido Radical Complexes Supported by a Dianionic Pentadentate Ligand.” Chem. Sci. 2016, 7, 5939-5944.
Perfluorarylboranes and Boron-Nitrogen heterocycles
Our group has a long-standing interest in organoborane chemistry and our investigations have fallen into one of two themes.
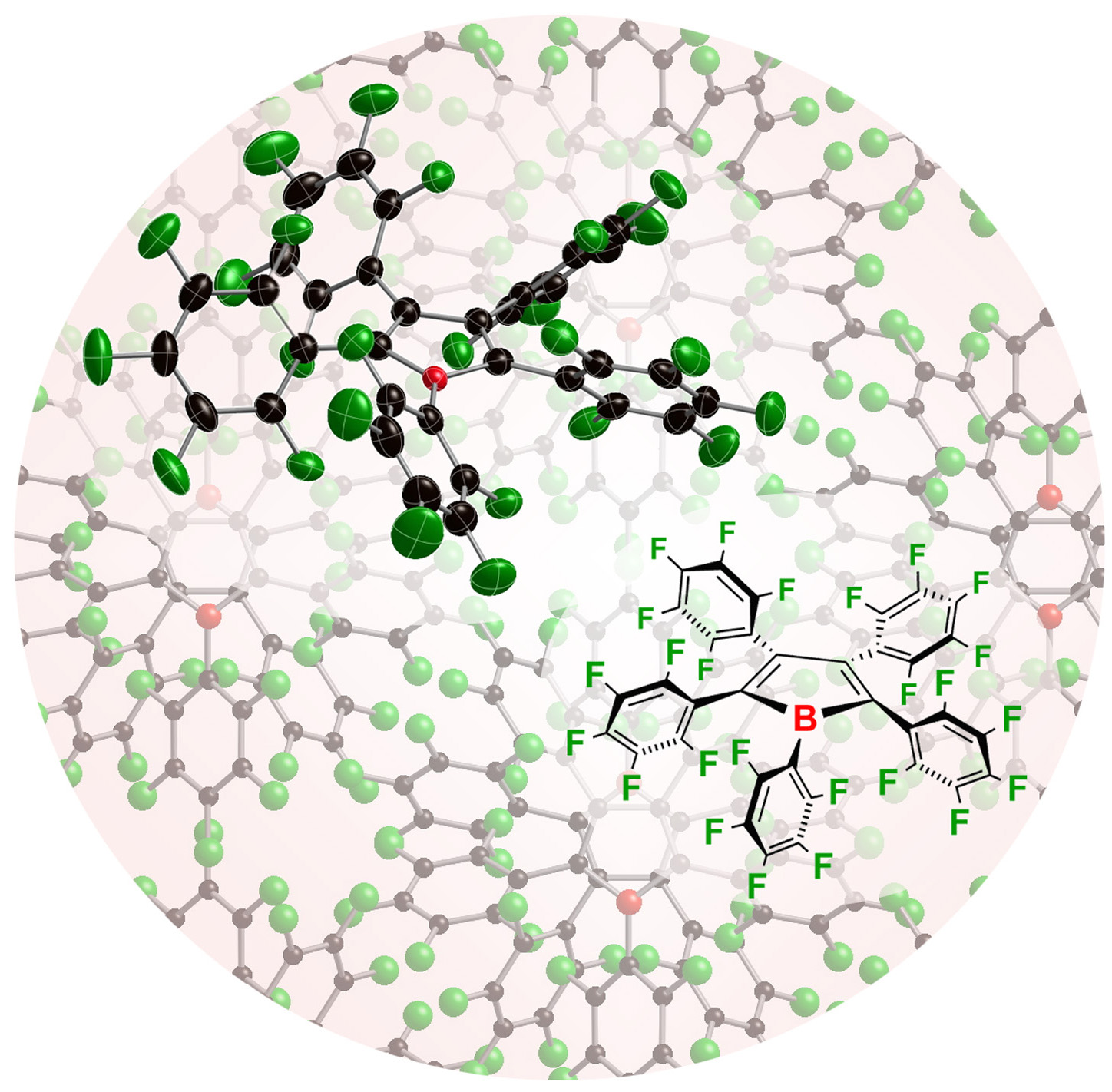
Perfluoroarylboranes are a class of highly Lewis acidic boranes that are also remarkably robust due to the kinetic and thermodynamic stability of the B-C bonds. We have introduced a number of new perfluorarylboranes and contributed seminal studies on the use of the parent borane, B(C6F5)3, as a catalyst for a variety of transformations.
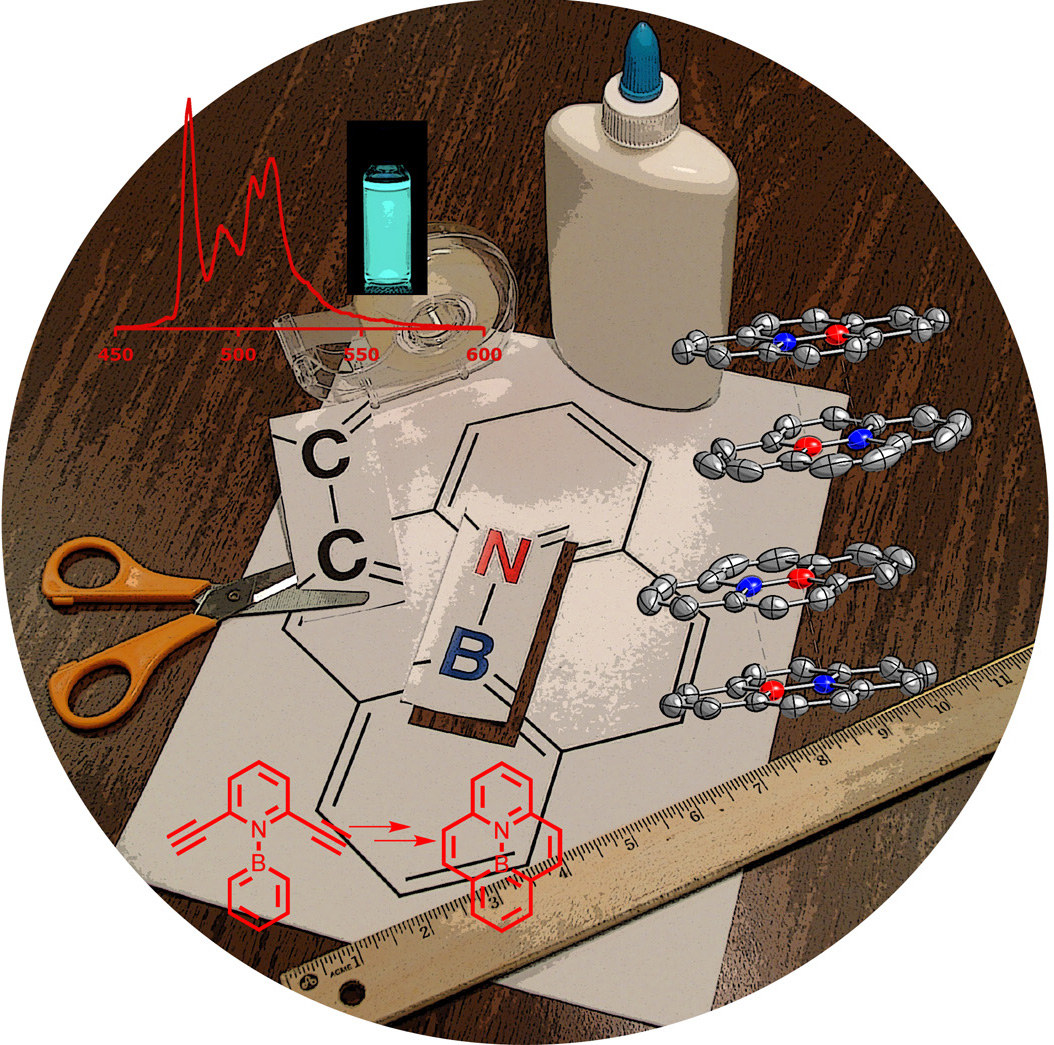
In a second theme, we have introduced a number of BN analogs of polycyclic aromatic hydrocarbons, in which “CC” units are replaced by “BN”. This influences the photophysical and redox properties of the compounds that provide advantages in the application of these compounds as organic materials in OLED and other devices.
Currently we are using electrophilic borylation to generate new BN conjugated systems and evaluating their materials properties.
Key papers:
Patrick, E. A.; Piers, W. E. “Twenty-five years of bis-pentafluorophenyl borane: a versatile reagent for catalyst and materials synthesis” Chem. Commun. 2020, 56, 841-853.
Morgan, M. M.; Nazari, M.; Pickl, T.; Rautiainen, J. M.; Tuononen, H. M.; Piers, W. E., Welch, G. C., Gelfand, B. S. “Boron-Nitrogen Doped Dihydroindeno[1,2-b]fluorene Derivatives as Acceptors in Organic Solar Cells” Chem. Commun. 2019, 55, 11095-11098.
Berkefeld, A.; Piers, W. E.; Parvez, M. “Tandem Frustrated Lewis Pair/Tris-Pentafluorophenylborane Catalyzed Deoxygenative Hydrosilation of Carbon Dioxide.” J. Am. Chem. Soc. 2010, 132, 10660-10661.
Houghton, A. Y.; Piers, W. E., Tuononen, H. “Direct observation of a borane-silane complex involved in frustrated Lewis pair mediated hydrosilation of olefins.” Nat. Chem. 2014, 6, 983-988.
Bosdet, M. J. D.; Piers, W. E.; Sorensen, T. S.; Parvez, M. “11-Aza-12-Borapyrenes: Heterocyclic Analogues of Pyrene with Internalized BN Moieties.” Angew. Chem. Int. Ed. 2007, 46, 4940-4943.
Neue, B.; Araneda, J. F.; Piers, W. E.; Parvez, M. “BN-Dibenzo[a,o]picenes: Stable Heptacene Analogs.” Angew. Chem. Int. Ed. 2013, 52, 9966-9969.
Morgan, M. M.; Piers W. E. “Efficient Synthetic Methods for the Installation of Boron-Nitrogen Bonds in Conjugated Organic Molecules.” Dalton Trans. 2016, 45, 5920-5924.
Electrocatalytic reduction of CO2
The imperatives of climate change have spurred worldwide efforts for the development of processes that would utilize CO2 on a large scale as a feedstock for usable fuels. Not only are new CO2 reduction catalysts required, but they need to be incorporated into robust devices that utilize renewable energy to power them.
In a multidisciplinary project funded by the University of Calgary’s Global Research Initiative in Sustainable Low Carbon Unconventional Resources, we are designing new molecular CO2 reduction catalysts for anchoring to carbon-based conducting electrodes and incorporation into efficient and long-lived devices.
New ligands for use in molecular electrocatalysts based on first row transition metals are being designed, synthesized and evaluated as part of this project.
Key papers:
Koenig, J. D. B.; Willkomm, J.; Roesler, R.; Piers, W. E.; Welch, G. C. “Electrocatalytic CO2 reduction at lower overpotentials using iron(III) tetra(miso-thienyl)porphyrins” ACS Appl. Energy Mater. 2019, 2, 4022-4026.
Willkomm, J.; Bertin, E., Atwa, M.; Birss, V. I.; Piers, W. E. “Grafting of a molecular rhenium CO2 reduction catalyst onto colloidal imprinted carbon” ACS Appl. Energy Mater. 2019, 2, 2414-2418.
Dubrawski, Z.; Heidebrecht, J. E.; Puerta Lombardi, B. M.; Hyla, A.; Willkomm, J.; Radford, C. L.; Lin, J.-B.; Welch, G. C.; Ponnurangam, S.; Roesler, R.; Prokopchuk, D. E.; Piers, W. E. “Ligand-Centered Electrochemical Processes Enable CO2 Reduction with a Nickel Bis(triazapentadienyl) Complex” Sustainable Energy Fuels 2019, 3, 1172-1181.